The Role of Radiopharmaceuticals in the Evaluation of Heart Function
editor’s note: Please look at the article from Circulation entitled ‘Radiation Dose to Patients From Cardiac Diagnostic Imaging‘ comparing radiation absorbed doses broken down by tissue and organ type.
There are two main categories of radiopharmaceuticals: Myocardial Imaging Agents and Blood Pool Imaging agents. Myocardial imaging agents may include markers of perfusion, hypoperfusion, necrosis, and metabolism. Imaging techniques include Planar, gated or non-gated Single Photon Computed Tomography, or Positron Emission Tomography. Blood Pool Imaging techniques include gated and non-gated first pass imaging, and planar or SPECT gated blood pool techniques for the evaluation of left and right ventricular function and cardiac output.
Myocardial imaging agents are classified as:
- “hot spot” infarct-avid agents
- “cold spot” markers of hypoperfused tissue
- adrenergic neuronal markers
Blood pool imaging agents include:
- tagged autologous red blood cells
- radiolabeled microspheres
Imaging Myocardial Necrosis with Infarct-Avid agents
It is well established that the size of the defect as demonstrated by nuclear scintigraphy is a predictor of patient outcome. There is a very positive correlation between infarct size and mortality. Two imaging agents used for imaging acute myocardial necrosis are absorbed into newly infarcted myocardial tissue. These “hot-spot” imaging agents include:
- 99mTc Pyrophosphate
- Indium-111-labeled antimyosin antibodies
- historical note: infarct-avid radiopharmaceuticals that are no longer used in Nuclear Medicine include: Mercury-203 chlormerodrin, Tc99m tetracycline, Tc99m gluceptate, Tc99m medronate
The 99mTc Pyrophosphate scan was an important tool back when Planar Thallium or MUGA
scan protocols were still being perfected, prior to the development of gated
SPECT imaging and technetium-based radiopharmaceuticals. It was a simple,
very inexpensive method employed to find new and evolving myocardial infarctions
in patients coming to the Emergency Department with chest pain.
Tc99m Pyrophosphate
Preparation and pharmacokinetics: Technetium labeled pyrophosphate is prepared the same way bone imaging agents are prepared. The technetium-99m forms a chelate with the pyrophosphate molecule. The pharmacokinetics is similar to diphosphonate (MDP). Skeletal uptake is about 40-50% of the injected dose; the cumulative urinary excretion at 24 hours is about 60%. Demonstrable uptake is evident after 12 hours, with maximum localization in infarcted tissue 24 – 48 hours after acute infarction. Image 3-4 hours post injection to allow for more complete clearance from the blood. Mechanisms of Localization: Damage to the myocyte (infarction) leads to loss of integrity of the cell membrane, allowing cell contents to escape and infiltration of substances ordinarily excluded. Following cell death in acute MI there is an influx of calcium and the formation of various calcium phosphate complexes. These microcrystalline deposits act as sites for Tc99m pyrophosphate uptake. Some binding may also occur on denatured macromolecules.
Some residual blood flow around the infarct is necessary to deliver tracer to the injured tissue.
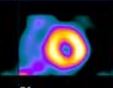
The tracer diffuses into the necrotic tissue and is bound, with the highest uptake uptake at the periphery of the infarct. A characteristic ring or donut pattern is sometimes seen in large infarctions when neither direct flow nor diffusion is present.
15-25 mCi is administered IV, with imaging begun 3-4 hours later. A combination of Planar and SPECT imaging techniques may be used to interpret the exam. Using a high resolution collimator, 500K views including the anterior, 45 LAO, 70 LAO and left lateral views of the chest are acquired. Acquire ECT as you would a bone SPECT centered on the thorax.
The optimum time to apply the procedure is 24 to 48 hours post acute infarction.
NOTES:
-
- In ~30% of patients, myocardial infarction images will remain abnormal indefinitely after transmural infartion, particularly in those in whom LV aneurysms develop
- Transmural infarction is detected with >90% sensitivity and can usually be accurately localized by evaluation of anterior, lateral, and oblique views
False Negative Tc99m Pyrophosphate Studies: This test is less accurate in diagnosing subendocardial infarction. In these cases, uptake is diffuse rather than focal.False Positive Tc99m PYP Studies:
FOCAL: old myocardial infarction (persistent positivity), calcification (valvular, pericardial), ventricular aneurysm, costal cartilage calcification
DIFFUSE: unstable angina, myocarditis, pericarditis, cardiomyopathy, amyloidosis, radiation therapy, persistent blood pool activity, adriamycin therapy
In-111 Antimyosin Antibody Imaging
An alternative approach for detection of acute necrosis uses an antibody raised against the heavy chain of cardiac myosin. Cardiac myosin is a key protein of the heart’s contractile apparatus. Loss of integrity of the cell membrane in irreversibly damaged cells permits antimyosin to enter the damaged cell and combine with the antigen, localizing in areas of acute necrosis.
In-111 antimyosin is a monoclonal antibody that binds to cardiac myosin exposed upon cell death. Maximum uptake occurs in regions of lowest flow, and primarily in necrotic areas. In-111 antimyosin is particularly valuable in evaluation of myocarditis and heart transplant rejection.
Antimyosin antibody is administered as an Fab fragment, which has a blood clearance half-time of 10 to 12 hours compared with about 18 to 20 hours for the intact antibody. The antibody fragment is labeled with 111-In using a bifunctional chelate. Localization of antimyosin can be seen within hours of occlusion and occurs in most patients with acute infarction if the antibody is administered within 10 to 14 days of the event. Thereafter the incidence of localization decreases, such that after 9 months, patients with acute infarction rarely localize antimyosin at the site of necrosis.
By performing dual-isotope tomographic imaging with In-111 monoclonal antibodies and Tl-201, infarct size and percentage of infarcted myocardium can be estimated accurately. Furthermore, the antimyosin images can then be superimposed on the perfusion images to distinguish between viable and necrotic tissue.
Drawbacks of In-111 antimyosin are:
-
-
- 1. Blood pool contamination and interference from liver activity with In-111
-
-
-
- 2. Suboptimal imaging characteristics (gamma-emission 170 and 247 keV, half-life of 68 hours),
-
-
-
- 3. The late moment of reliable infarct definition at about 48 hours post-infarction
-
SIGNIFICANCE OF NUCLEAR SCINTIGRAPHY WITH RESPECT TO RISK AND PROGNOSIS
-
-
- Risk stratification and prognosis following MI:
It is well established that the size of the defect as demonstrated by nuclear scintigraphy is a predictor of patient outcome. There is a very positive correlation between infarct size and mortality. - Assessment of thrombolytic therapy: Prior to thrombolytic therapy, scintigraphy can demonstrate ischemic areas of the heart and the watershed distal to the coronary thrombus. Following successful clot lysis with reestablishment of perfusion, a post-therapy perfusion scintigram is used to document the degree of reperfusion. Due to complications contributed by edema and hemorrhage, follow-up imaging 1-2 weeks after thrombolysis may be indicated.
- Myocardial Viability: Stunning versus hibernation
“Stunned” and “hibernating myocardium” describes viable myocardium receiving an impaired blood supply. A myocardial segment is “stunned” if it takes up the radiotracer but is akinetic. “Hibernating” refers to severe, chronically ischemic tissue that is viable but appears cold on thallium imaging and nonfunctional on ventriculography or echography. PET imaging with F-18 FDG has been shown to detect such tissue and correctly indicate its viability.
- Risk stratification and prognosis following MI:
-
MYOCARDIAL PERFUSION IMAGING: EVALUATION OF MYOCARDIAL FUNCTION AND THERAPY Nuclear imaging techniques noninvasively map regional perfusion of viable tissue.
The scintigram depicts two sequential events:
-
-
- delivery of tracer to the myocardium;
- visualization of viable, metabolically active myocardial cells that localize the tracer.
-
Studies obtained at rest are used for the diagnosis of acute MI, determining the presence and extent of infarction (or the residual mass of viable myocardium), and to assess therapeutic interventions (angioplasty and thrombolysis).
There are numerous and subtle differences between the different radiopharmaceuticals for myocardial perfusion imaging to evaluate myocardial ischemia and viability. Imaging modalities include planar, SPECT, MCD or PET. Radiopharmaceuticals approved for myocardial perfusion imaging
-
-
- Thallium 201 (THALLOUS CHLORIDE)
- 99m Tc Sestamibi (methoxyisobutyl isonitrile) CARDIOLITE
- 99m Tc Tetrofosmin MYOVIEW
- 18 FDG (2-deoxy-2 [18F] Fluoro-D-Glucose)
- I-123 MIBG (metaiodobenzylguanidine)- neuronal imaging agent
- Nitrogen-13 Ammonia
- Rubidium-82
-
(Historical Note: before the ECT imaging system was a common fixture in the nuclear lab, 99mTc-Teboroxime, or Cardiotec, was widely used as a perfusion agent. 99mTc-Teboroxime cleared rapidly from the blood and was retained for a very short time in the myocardium. Planar or tomographic imaging had to begin at 2 minutes and be completed within 5-10 minutes following tracer administration. Separate rest and stress injections were given 60-90 minutes apart.)
COLD-SPOT MARKERS OF HYPOPERFUSED TISSUE A “cold-spot” marker is absorbed into living myocytes which receive adequate blood flow. Scar tissue never takes up the radiotracer and remains “cold” on post-stress and resting baseline images.
Cold-spot markers of hypoperfusion include:
-
-
- 201-Thallous Chloride
- Tc99m Sestamibi (Cardiolite)
- Tc99m Tetrofosmin (Myoview)
- Tc99m Teboroxime
- Tc99m Noet
-
201 -Thallous Chloride For decades, Thallium-201 Chloride was the agent of choice to assess the effect of CAD on regional myocardial perfusion, function and viability, and predict patient prognosis. 201-Thallous chloride has excellent physical properties for medical evaluation of myocardial cell viability and tissue function.
Thallium-201, however, possesses suboptimal imaging energy in terms of its use with the gamma camera. Useful imaging photons are easily scattered and absorbed in soft tissue, and rapid redistribution creates logistic problems. Increasing the patient’s injected dose (in millicuries) should not exceed 7 mCi due to the radiation exposure to the patient by virtue of its 73 hour half-life.
Thallium is a cyclotron product, decaying by electron capture to mercury 201. The photons available for imaging are mercury L-alpha and K-beta characteristic x-rays in the range of 69 to 83 keV (87% abundant) and thallium gamma rays of 167 keV (10% abundant) and 135 keV
(3% abundant).
Major contaminants in
the 201-thallous chloride solution may include:
200Tl t1/2= 27 hrs, gamma1= 350 keV (0.3%)
gamma2= 1.6 meV
202Tl t1/2=11.8 days, gamma1= 439 keV (1.2%)
203Pb (0.02%)
(Expires 9 days post manufacture)
Mechanism of localization and pharmacokinetics Thallium behaves in its organ and tissue distribution much like Potassium and needs the sodium/potassium/ ATPase pump for active transport into cells. A major advantage of Tl-201 for myocardial perfusion imaging is its 88% extraction rate first pass during transit through the myocardial capillary bed, under conditions of normal coronary flow. Peak uptake in the myocardium occurs in 10-20 minutes following injection (in normal subjects ~ 5% of the administered dose localizes in the myocardium).
Thallium is taken up in all cellular, metabolically active tissues in the body with the exception of the brain. It does not cross the normal (intact) blood brain barrier. Activity on resting studies is normally seen in the liver and GI tract, thyroid and salivary glands, the kidneys and skeletal muscle.
Increased lung activity at rest has been linked to underlying lung disease, including interstitial and inflammatory processes, and increased uptake is frequently seen in smokers.
Tc99m MYOCARDIAL PERFUSION IMAGING AGENTS
-
-
- Tc99m Sestamibi (Cardiolite)
- Tc99m Tetrofosmin (Myoview)
- Tc99m Noet
- Tc99m Teboroxime
-
The technetium-labeled agents can be substituted for thallium in the rest and stress evaluation of myocardial perfusion. These agents are very different in behavior compared with Thallous Chloride. The isotope chart on the following page gives a detailed comparison between the Tc99m compounds used for SPECT myocardial perfusion imaging.
The technetium-labeled agents are lost slowly from the myocardium, with comparable clearance from normal and ischemic tissue. Since Tc99m-labeled tracers are essentially fixed in the cells, separate injections are required for the rest and the stress portions of the examination. Optimally, the rest and stress exams are performed on separate days. Same-day studies use as little as 8-10 mCi for the first dose and up to 45 mCi for the second. The second injection should contain at least 2.5 to 3 times the resting dose. The doses are adjusted for the weight of the patient. When possible, the injections should be separated by a three hour delay, though time constraints may not allow for this.
POSITRON EMISSION TOMOGRAPHY Cardiologists are using PET in their protocols to
evaluate myocardial viability, assess the potential for successful coronary revascularization,
and for early diagnosis of atherosclerosis. Overview of Metabolic Imaging Radiolabeled metabolic substrates give us a look at the in-vivo myocardial biochemistry and help evaluate therapeutic interventions for acute ischemic states. The positron emitting isotope as a tracer is crucial in accurately imaging and quantifying local tissue perfusion, blood volume, glucose and oxygen consumption and fractional extractions of various metabolites and substances.
Metabolic imaging may be most useful in patients with severely impaired regional and global function, in the case of F-18 FDG imaging of hibernating myocardial tissue. Advantages of PET:
-
-
- electrical collimation unique to PET yields high spatial resolution and high-count density images
- attenuation correction of cardiac PET is routinely performed
- stress and rest perfusion protocols may be completed in less time than with SPECT protocols
- PET perfusion is always performed with pharmacologic stress
- measures blood flow and metabolic rates of a variety of energy substrates in vivo (quantitatively)
- an unlimited number of positron-emitting tracers is available to evaluate specific aspects of myocardial tissue function
-
ON THE HORIZON: 123-I BMIPP SPECT IMAGING Iodofiltic Acid with I-123 is a new SPECT agent currently undergoing multicenter clinical trials. 123-I BMIPP has been demonstrated to improve initial diagnosis in Emergency Department chest pain patients with suspected Acute Coronary Syndromes. The agent, ß-methyl-p-[123I]-iodophenyl-pentadecanoic acid, may help physicians identify patients who may be at risk for a cardiac event.
Free fatty acids are the preferred substrate for high-energy adenosine triphosphate production in the normal myocardium. In the setting of myocardial ischemia, high-energy adenosine triphosphate production shifts from fatty acid metabolism to glucose utilization. This suppression of fatty acid metabolism may persist long after the resolution of the perfusion abnormality and ischemia, a phenomenon that has been referred to as ischemic memory.
BMIPP is a methyl branched-chain fatty acid that is not easily metabolized and thus is retained in myocardial cells. Clinical studies have demonstrated persistent reductions in BMIPP uptake long after resolution of ischemic symptoms. Therefore, BMIPP imaging may extend the window of time for identification of myocardial
ischemia after symptom resolution, even after restoration of myocardial blood flow.
Radiopharmaceutical developer Molecular Insight Pharmaceuticals of Cambridge, MA, is developing the molecular imaging agent under the brand name Zemiva. BMIPP is a methyl fatty acid that is retained by myocardial cells. Previous research has found that persistent reductions in BMIPP uptake can be present more than a day after ischemic symptoms have subsided and may extend the time physicians have to diagnose myocardial ischemia.
Related Articles on BMIPP: